Research
The focus of my research is the chemistry of persistent open-shell organic molecules (mono- and poly-radicals) and their applications in materials science. This work is interdisciplinary and spans the three-way interface between chemistry, physics and materials science. The primary goal of my research is to design, synthesize and characterize the molecular and solid state properties of stable radicals composed exclusively of light elements (either organic or main group). The long-term goal is to generate materials that can be incorporated in electronic and photonic devices for applications in quantum computing and energy conversion. These light-weight materials have the potential to reduce the size, weight, cost and power consumption of devices and simultaneously improve their efficiency and enhance their functionality. Organic and main group radicals are ideal building blocks for such materials owing to their unique physical properties (magnetic, optical, transport) which can be tailor-made to exhibit desired behaviors. This requires an understanding of the relationships between molecular structure and properties. My research efforts will concentrate on thorough studies of these relationships to unravel the rules that control electron transfer/transport events, intra- and inter-molecular exchange-coupling interactions and electron excitation/charge transfer in stable radicals.
Dr. Christos P. Constantinides leads an interdisciplinary research program in synthetic and physical organic chemistry, with two core projects centered on the design and application of stable organic radicals.
The first project focuses on the development of novel radical-based polarizing agents for Dynamic Nuclear Polarization (DNP) enhanced Magic Angle Spinning (MAS) Nuclear Magnetic Resonance (NMR) spectroscopy. This work seeks to overcome limitations in current DNP performance by synthesizing air-stable, metal-free radicals—primarily Blatter-type and perchlorotriphenylmethyl (PTM) radicals—with tailored electronic structures and spin distribution. These compounds are designed to improve polarization efficiency under MAS conditions and broaden the scope of hyperpolarization for biochemical and materials applications. The project integrates synthetic chemistry, EPR spectroscopy, and collaborations with Ames National Laboratory to evaluate DNP performance and develop structure–property relationships that inform radical design.
The second project explores the electronic and magnetic properties of π-extended organic radicals for spintronics applications. The Constantinides group investigates how molecular design influences intramolecular spin coupling, charge transport, and magnetic exchange in solid-state assemblies of stable radicals. Of particular interest are dimeric and polymeric motifs based on 1,2,4-benzotriazin-4-yl frameworks (Blatter radicals) that exhibit tunable ferromagnetic interactions. The goal is to establish synthetic platforms that enable the integration of organic radicals into spintronic devices, contributing to the development of lightweight, low-power alternatives to traditional inorganic components.
Together, these projects aim to bridge fundamental organic synthesis with cutting-edge applications in spectroscopy and molecular electronics, while providing a rich training environment for undergraduate researchers.
Thiazyl (S,N-rich) radicals
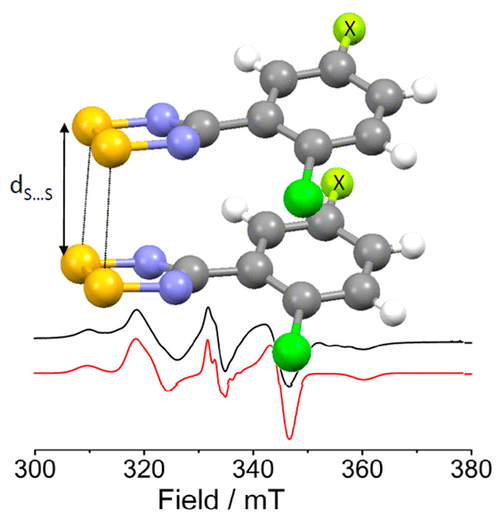
Evaluation of different supramolecular synthons on the crystal packing of 1,2,3,5-dithiadiazolyls (DTDAs). This class of main-group radicals have the tendency to dimerize in the solid-state via a multicenter two electron interaction quenching any potential magnetic and/or conducting properties. We have synthesized a number of DTDAs and characterized them by means of X-ray diffraction, SQUID magnetometry, EPR spectroscopy and computational studies in an effort to identify substituents that could inhibit Peierls’ distortion in their 1D π-stacks.
Benzo[e][1,2,4]triazin-4-yl radicals (aka Blatter radicals)
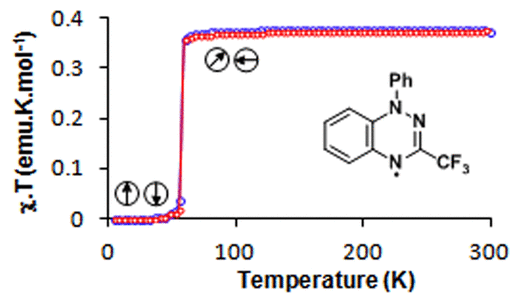
A new sub-family of hydrazyl organic radicals. 1,2,4-Benzotriazin-4-yls although first prepared in the late 1960s by Blatter they hardly received any attention compared to their closely related verdazyls despite their exceptional air and moisture stability. A number of different synthetic routes to 1,2,4-benzotriazinyls have been developed and many derivatives prepared to examine their solution (CV, EPR) and solid-state properties (X-ray, SQUID, EPR). My current research activities are focused in the preparation of multi-functional materials based on 1,2,4-benzotriazinyls.
Zwitterionic Polyazaacenes
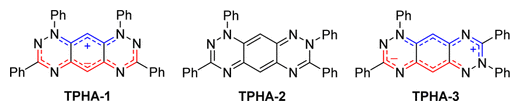
Acenes with zwitterionic biscyanine motifs are unusual, as their central arenes have lost their aromaticity by parting their π electrons. The first example, 5,7-diphenyl-5H,12H-quinoxalino[2,3-b]phenazine (DPTAP, a.k.a. diphenylisofluorindine) was reported as early as 1896, but at that time the exact electronic nature of the compound was unclear. While these compounds were of interest as potential textile dyes, little more was reported until 1998 when Wudl prepared the zwitterionic biscyanine of tetraphenylhexaazaanthracene TPHA-1. We have prepared and currently working on a range of new polyazaacene zwitterionic biscyanine compounds with interesting optical properties.